 |
PDBsum entry 1a9c

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Gtp cyclohydrolase i (c110s mutant) in complex with gtp
|
|
Structure:
|
 |
Gtp cyclohydrolase i. Chain: a, b, c, d, e, f, g, h, i, j, k, l, m, n, o. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 562. Organ: brain. Expressed in: escherichia coli. Expression_system_taxid: 562. Other_details: homologous expression
|
|
Biol. unit:
|
 |
 Homo-Decamer (from PDB file)
Homo-Decamer (from PDB file)
|
|
Resolution:
|
 |
|
2.90Å
|
R-factor:
|
0.206
|
R-free:
|
0.288
|
|
|
Authors:
|
 |
G.Auerbach,H.Nar,A.Bracher,A.Bacher,R.Huber
|
|
Key ref:
|
 |
G.Auerbach
et al.
Gtp cyclohydrolase I in complex with gtp at 2.1 a resolution.
To be published,
.
|
 |
|
Date:
|
 |
|
04-Apr-98
|
Release date:
|
11-May-99
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0A6T5
(GCH1_ECOLI) -
GTP cyclohydrolase 1 from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
222 a.a.
221 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.4.16
- Gtp cyclohydrolase i.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Folate Biosynthesis (early stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP + H2O = 7,8-dihydroneopterin 3'-triphosphate + formate + H+
|
 |
 |
 |
 |
 |
GTP
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
H2O
|
=
|
7,8-dihydroneopterin 3'-triphosphate
|
+
|
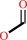 formate
formate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |

