Assemblies
Assembly Name:
Cellular tumor antigen p53
Multimeric state:
monomeric
Accessible surface area:
9974.19 Å2
Buried surface area:
279.4 Å2
Dissociation area:
0
Å2
Dissociation energy (ΔGdiss):
0
kcal/mol
Dissociation entropy (TΔSdiss):
0
kcal/mol
Symmetry number:
1
PDBe Complex ID:
PDB-CPX-138105
Assembly Name:
Cellular tumor antigen p53
Multimeric state:
monomeric
Accessible surface area:
10237.81 Å2
Buried surface area:
489.43 Å2
Dissociation area:
0
Å2
Dissociation energy (ΔGdiss):
0
kcal/mol
Dissociation entropy (TΔSdiss):
0
kcal/mol
Symmetry number:
1
PDBe Complex ID:
PDB-CPX-138105
Macromolecules
Chains: A, B
Length: 219 amino acids
Theoretical weight: 24.53 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
Pfam: P53 DNA-binding domain
InterPro:
Length: 219 amino acids
Theoretical weight: 24.53 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
- Canonical:
 P04637 (Residues: 94-312; Coverage: 56%)
P04637 (Residues: 94-312; Coverage: 56%)
Pfam: P53 DNA-binding domain
InterPro:
- p53 tumour suppressor family
- p53/RUNT-type transcription factor, DNA-binding domain superfamily
- p53-like transcription factor, DNA-binding domain superfamily
- p53, DNA-binding domain



![The deposited structure of PDB entry <span class='highlight'>5aok</span> coloured by chain, front view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 5aok coloured by chain, front view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_deposited_chain_front_image-200x200.png)
![The deposited structure of PDB entry <span class='highlight'>5aok</span> coloured by chain, side view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 5aok coloured by chain, side view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_deposited_chain_side_image-200x200.png)
![The deposited structure of PDB entry <span class='highlight'>5aok</span> coloured by chain, top view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 5aok coloured by chain, top view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_deposited_chain_top_image-200x200.png)
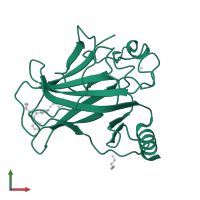
![Monomeric assembly 1 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, front view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 1 of PDB entry 5aok coloured by chemically distinct molecules, front view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_1_chemically_distinct_molecules_front_image-200x200.png)
![Monomeric assembly 1 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, side view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 1 of PDB entry 5aok coloured by chemically distinct molecules, side view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_1_chemically_distinct_molecules_side_image-200x200.png)
![Monomeric assembly 1 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, top view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 1 of PDB entry 5aok coloured by chemically distinct molecules, top view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_1_chemically_distinct_molecules_top_image-200x200.png)
![Monomeric assembly 2 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, front view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 2 of PDB entry 5aok coloured by chemically distinct molecules, front view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_2_chemically_distinct_molecules_front_image-200x200.png)
![Monomeric assembly 2 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, side view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 2 of PDB entry 5aok coloured by chemically distinct molecules, side view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_2_chemically_distinct_molecules_side_image-200x200.png)
![Monomeric assembly 2 of PDB entry <span class='highlight'>5aok</span> coloured by chemically distinct molecules, top view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>1 copy of <span class='highlight'>Cellular tumor antigen p53</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>5-[2-cyclopropyl-5-(1H-pyrrol-1-yl)-1,3-oxazol-4-yl]-1H-1,2,3,4-tetrazole</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Monomeric assembly 2 of PDB entry 5aok coloured by chemically distinct molecules, top view.](https://www.ebi.ac.uk/pdbe/static/entry/5aok_assembly_2_chemically_distinct_molecules_top_image-200x200.png)