EMD-5525
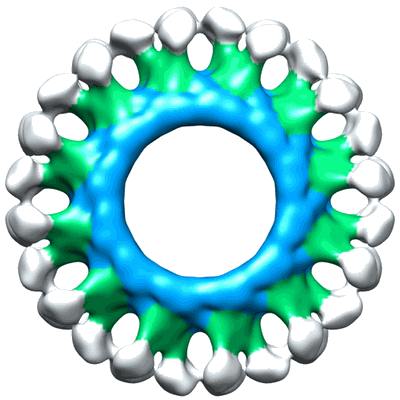
Electron cryo-microscopy of ABC BmrA in 12-fold symmetry rings
EMD-5525
Single-particle23.0 Å
 Deposition: 02/11/2012
Deposition: 02/11/2012Map released: 21/11/2012
Last modified: 14/05/2014
Concentration: 0.1
mg/mL
Buffer
pH: 7.8
Details: 50mM HEPES, 100mM NaCl
Details: 50mM HEPES, 100mM NaCl
Grid
Details: Ted Pella inc. holey formvar grid
Vitrification
Cryogen name: ETHANE
Instrument: HOMEMADE PLUNGER
Method: Blot for 3 seconds before plunging.
Details: Specimen linked to a Ni++-NTA-DOGS/DOPC lipid monolayer
Instrument: HOMEMADE PLUNGER
Method: Blot for 3 seconds before plunging.
Details: Specimen linked to a Ni++-NTA-DOGS/DOPC lipid monolayer
Microscope: FEI/PHILIPS CM200FEG
Illumination mode: FLOOD BEAM
Imaging mode: BRIGHT FIELD
Electron source: FIELD EMISSION GUN
Acceleration voltage: 200 kV
Nominal CS: 2.26 mm
Nominal defocus: 2.6 µm - 5.0 µm
Nominal magnification: 50000.0
Calibrated magnification: 50000.0
Specimen holder model: GATAN LIQUID NITROGEN
Illumination mode: FLOOD BEAM
Imaging mode: BRIGHT FIELD
Electron source: FIELD EMISSION GUN
Acceleration voltage: 200 kV
Nominal CS: 2.26 mm
Nominal defocus: 2.6 µm - 5.0 µm
Nominal magnification: 50000.0
Calibrated magnification: 50000.0
Specimen holder model: GATAN LIQUID NITROGEN
Image Recording
[1]
Detector category:
FILM
Detector model: KODAK SO-163 FILM
Scanner: NIKON COOLSCAN
Number of real images: 102
Average electron dose per image: 15 e/Å2
Bits per pixel: 8.0
Detector model: KODAK SO-163 FILM
Scanner: NIKON COOLSCAN
Number of real images: 102
Average electron dose per image: 15 e/Å2
Bits per pixel: 8.0
Details: Xmipp CL2D classification and Projection matching refinement with Invert Fourier 3D reconstruction
Final
reconstruction
Resolution: 23.0
Å
(
BY AUTHOR)
Resolution method: FSC 0.5 CUT-OFF
Number of images used: 1188
Algorithm: OTHER
Details:
Resolution method: FSC 0.5 CUT-OFF
Number of images used: 1188
Algorithm: OTHER
Details:
⌯ Applied Symmetry
Point group:
D12
Software
[1]
| Name | Version | Details |
|---|---|---|
| Xmipp | - | - |
CTF correction
Details:Micrograph based
Format: CCP4
Data type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
Annotation details: Reconstruction of BmrA in a lipidic environment. Reconstruction is D12 symmetrized. 24 homodimers of BmrA are inserted into a lipid bilayer.
Details: ::::EMDATABANK.org::::EMD-5525::::
Data type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
Annotation details: Reconstruction of BmrA in a lipidic environment. Reconstruction is D12 symmetrized. 24 homodimers of BmrA are inserted into a lipid bilayer.
Details: ::::EMDATABANK.org::::EMD-5525::::
⬡ Geometry
| X | Y | Z | |
|---|---|---|---|
| Dimensions | 200 | 200 | 200 |
| Origin | 0 | 0 | 0 |
| Spacing | 200 | 200 | 200 |
| Voxel size | 2.50 Å | 2.50 Å | 2.50 Å |
Contour list
| Primary | Level | Source |
|---|---|---|
| True | 0.029 | AUTHOR |
