Function and Biology Details
Reactions catalysed:
4-amino-2-methyl-5-diphosphomethylpyrimidine + 2-((2R,5Z)-2-carboxy-4-methylthiazol-5(2H)-ylidene)ethyl phosphate = diphosphate + thiamine phosphate + CO(2)
ATP + 4-methyl-5-(2-hydroxyethyl)thiazole = ADP + 4-methyl-5-(2-phosphonooxyethyl)thiazole
Biochemical function:
Biological process:
Cellular component:
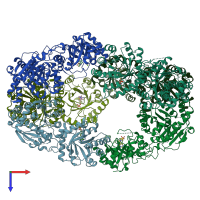
Structure analysis Details
Assembly composition:
homo hexamer (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-179633 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: