Assemblies
Assembly Name:
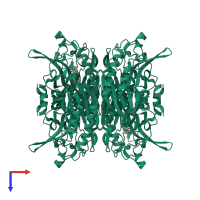
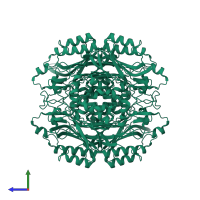
N-carbamoyl-D-amino acid hydrolase
Multimeric state:
homo tetramer
Accessible surface area:
38371.04 Å2
Buried surface area:
14603.52 Å2
Dissociation area:
1,933.87
Å2
Dissociation energy (ΔGdiss):
1.76
kcal/mol
Dissociation entropy (TΔSdiss):
15.06
kcal/mol
Symmetry number:
4
PDBe Complex ID:
PDB-CPX-157995
Macromolecules
Chains: A, B
Length: 303 amino acids
Theoretical weight: 34.14 KDa
Source organism: Agrobacterium sp.
Expression system: Escherichia coli
UniProt:
InterPro:
CATH: Carbon-nitrogen hydrolase
SCOP: Carbamilase
Length: 303 amino acids
Theoretical weight: 34.14 KDa
Source organism: Agrobacterium sp.
Expression system: Escherichia coli
UniProt:
- Canonical:
 P60327 (Residues: 2-304; Coverage: 100%)
P60327 (Residues: 2-304; Coverage: 100%)
InterPro:
CATH: Carbon-nitrogen hydrolase
SCOP: Carbamilase