Assemblies
Assembly Name:
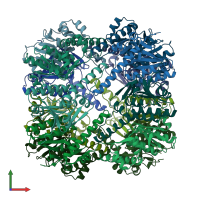
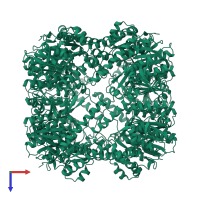
Endopeptidase ClpP complex
Multimeric state:
homo tetradecamer
Accessible surface area:
89876.74 Å2
Buried surface area:
57946.49 Å2
Dissociation area:
6,273.11
Å2
Dissociation energy (ΔGdiss):
69.86
kcal/mol
Dissociation entropy (TΔSdiss):
17.1
kcal/mol
Symmetry number:
14
PDBe Complex ID:
PDB-CPX-141326
Macromolecules
Chains: A, B, C, D, E, F, G, H, I, J, K, L, M, N
Length: 193 amino acids
Theoretical weight: 21.59 KDa
Source organism: Escherichia coli
Expression system: Not provided
UniProt:
Pfam: Clp protease
InterPro:
SCOP: Clp protease, ClpP subunit
Length: 193 amino acids
Theoretical weight: 21.59 KDa
Source organism: Escherichia coli
Expression system: Not provided
UniProt:
- Canonical:
 P0A6G7 (Residues: 15-207; Coverage: 93%)
P0A6G7 (Residues: 15-207; Coverage: 93%)
Pfam: Clp protease
InterPro:
- ATP-dependent Clp protease proteolytic subunit
- Clp protease proteolytic subunit /Translocation-enhancing protein TepA
- ClpP/crotonase-like domain superfamily
- ClpP, Ser active site
- ClpP, histidine active site
SCOP: Clp protease, ClpP subunit