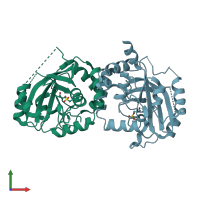
Assemblies
Assembly Name:
Assemblin
Multimeric state:
homo dimer
Accessible surface area:
18040.23 Å2
Buried surface area:
3037.82 Å2
Dissociation area:
1,518.91
Å2
Dissociation energy (ΔGdiss):
11.44
kcal/mol
Dissociation entropy (TΔSdiss):
12.76
kcal/mol
Symmetry number:
2
PDBe Complex ID:
PDB-CPX-147808
Macromolecules
Chains: A, B
Length: 256 amino acids
Theoretical weight: 28.22 KDa
Source organism: Human herpesvirus 5 strain AD169
Expression system: Escherichia coli
UniProt:
Pfam: Assemblin (Peptidase family S21)
InterPro:
CATH: Herpesvirus/Caudovirus protease domain
SCOP: Herpes virus serine proteinase, assemblin
Length: 256 amino acids
Theoretical weight: 28.22 KDa
Source organism: Human herpesvirus 5 strain AD169
Expression system: Escherichia coli
UniProt:
- Canonical:
 P16753 (Residues: 1-256; Coverage: 36%)
P16753 (Residues: 1-256; Coverage: 36%)
Pfam: Assemblin (Peptidase family S21)
InterPro:
CATH: Herpesvirus/Caudovirus protease domain
SCOP: Herpes virus serine proteinase, assemblin