Kim et al., (2007). A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways.
August 2013, model of the month by Vijayalakshmi Chelliah
Original model: BIOMD0000000149
Crosstalk interactions that couple distinct signalling pathways, despite their significant role in various cellular mechanisms, remain poorly understood due to their complex nature. Any changes in the coupling components result in significant functional shift of the system at a larger scale. For instance, mutational changes in individual proteins can cause fundamental functional changes in all the pathways they function in.
Several studies have identified the cause of abnormal activities of single pathway resulting from mutations of individual signalling proteins. But, the extensive crosstalk mechanism between pathways of the signalling system forms a complex network, which is rather not easy to understand by experimental approaches alone. Designing pathway models by integrating the relevant experimental individual pathway studies can identify key hidden mechanisms that can be tested experimentally and give insights to further findings.
The Wnt and ERK pathways are found to play an important role in colorectal cancers. The crosstalk between these two pathways have been identified by several groups. Kim et al. 2007 [1] have discovered a positive feedback loop embedded in the crosstalk between the Wnt and ERK pathways, by designing a pathway model constructed across integrating three experimental findings (refer to [1] for more details). The Wnt signalling pathway is one of the well-known oncogenic pathways, which is of particular importance in colorectal cancer. The pathway is normally regulated by β-catenin protein levels. A protein complex comprising axin, adenomatous polyposis coli (APC) andglycogenn synthase kinase 3β (GSK-3β) proteins binds β-catenin to promote phosphorylation of β-catenin by GSK-3β which leads to the degradation of β-catenin. Wnt induces the inhibition of GSK-3β, thereby elevating β-catenin levels and accumulation of β-catenin in the nucleus. Within the nucleus it associates with T-cell factor (TCF) to form a transcription factor that can drive proliferation. Colorectal cancer cells commonly have a constitutively high β-catenin level caused by inactivating mutations in APC or less frequently by mutations of the GSK-3β phosphorylation sites in β-catenin. This leads to aberrant accumulation of β-catenin and constitutive formation of the β-catenin/TCF transcription factor, which results in persistent proliferation.

Figure 2 A diagram summarising the hidden positive feedback mechanism between the Wnt and ERK pathways. Arrows denote activation and the blunted line indicates inhibition. See text for more details.Figure taken from [1].
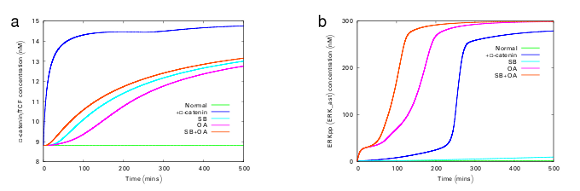
Figure 3 The effect of parameter perturbation on β-catenin and ERKpp levels under normal and mutated conditions. The plot was obtained by simulating BIOMD0000000149 by changing various parameter values. This figure is the reproduction of figure 3 of [1]. Refer to Figure 3 of [1] for parameter settings.
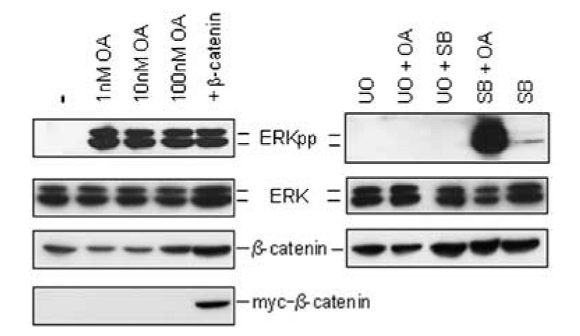
Figure 4 The effect of pharmacological perturbations on β-catenin and ERKpp levels. Figure taken from [1].
A signalling pathway system is normally activated upon an input stimuli, typically an extracellular growth factor. Apparently, this activation declines when the signal is removed in order to maintain the homeostasis in cellular systems. The model demonstrates this behaviour of the system, i.e. the upregulation of β-catenin/TCF complex and the ERKpp, returns to their basal levels after the Wnt signal is removed (Figure 5). However, if the β-catenin synthetic rate is elevated slightly above normal (two-fold of normal) or if the MEK/ERK phosphatase activities are reduced (three-fourths of normal), the output is completely different. It is observed that the system does not return to its inactivate state even after the Wnt signal disappears and remains in a persistently activated state, that is biologically proliferative state. The model also demonstrates that if the molecule X is not synthesized by β-catenin/TCF, the state transition cannot occur, and even will return to the inactivated state due to the breakdown of the Wnt/ERK positive feedback (Figure 6).
In summary, the model prediction suggest that the positive feedback between the Wnt and ERK pathways induces a state transition from an inactive to a constitutively activated state that may provide a chronic stimulus for cell proliferation that is maintained even in the absence of extracellular growth factor stimulation, and hence may contribute to the pathogenesis of cancer.

Figure 1 Wnt and ERK signalling pathway: Blue arrows indicate the interactions between the pathways. The image was implemented from [1]. Certain changes that is implemented in this figure (when compared to the original figure in the paper), is done referring to the figure in [2].
The ERK pathway is also a well-recognised major cellular proliferation signalling pathway that mediates proliferation in response to extracellular stimuli. The pathway is found constitutively activated in approximately 30% of all human cancers including colorectal cancer. The activation of this pathway through epidermal growth factor receptor (EGFR) is most thoroughly studied. Ras proteins activated upon extracellular stimuli, initiates the activation (phosphorylation) of series of kinases (Raf-1->MEK->ERK). Double phosphorylated ERK (ERKpp) translocate into the nucleus to phosphorylate other signalling molecules and transcription factors that participate in the regulation of proliferation. Mutation in Ras and B-Raf are found in colorectal cancers.
Kim et al. (2007) [1, BIOMD0000000149] have integrated the following experimental evidences into a pathway model. 1) Wnt signal activates Ras; 2) β-catenin/TCF activates Raf-1 through a yet unknown mechanism, here denoted as activator molecule X; 3) ERKpp phosphorylates GSK-3β and primes it for inhibition. Design of a pathway model by integrating the above experimental findings have identified a hidden positive feedback mechanism that leads to bistability in the system. The authors suggest that this can critically contribute to the pathogenesis of colorectal cancer. The diagram showing the Wnt and ERK signalling pathway is shown in (Figure 1) and the summarised positive feedback loop embedded within the two pathways is shown in (Figure 2).
The model describes the effects of activated ERK on the regulation of β-catenin/TCF levels as well as the effects of upregulated β-catenin/TCF on ERK activity. The model also simulates the effects of constitutive activation of the ERK or Wnt pathways. Levels of ERKpp and the β-catenin/TCF transcription factor were used as outputs of the respective pathways. Constitutive activation was mimicked by (a) assuming an enhanced expression of β-catenin, (b) using inhibitors of ERK phosphatases (okadaic acid; OA), (c) inhibitors of GSK-3β (SB216763; SB) or (d) a combination of OA+SB. These conditions functionally correspond to the constitutive activation of the Wnt pathway, ERK pathway or both. Figure 3 shows the simulation results of these mutated conditions, with respect to the normal condition. This illustrates that the positive feedback loop formed by the crosstalk induces a state transition from an inactive state to a constitutively activated state and maintain it. These predictions obtained by simulations, were also tested experimentally (Figure 4) and is qualitatively consistent with that of the model.

Figure 5 The effect of normal and mutated Wnt signalling on β-catenin and ERKpp levels. The plot was obtained by simulating BIOMD0000000149 by changing various parameter values. This figure is the reproduction of Figure 6 of [1]. Refer to Figure 6 of [1] for parameter settings.

Figure 6 Comparison of the Wnt and ERK signalling dynamics with and without crosstalk. The plot was obtained by simulating BIOMD0000000149 by changing various parameter values. This figure is the reproduction of Figure 7 of [1]. Refer to Figure 7 of [1] for parameter settings.
Bibliographic references
- Kim et al. A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways. Oncogene. (2007), 26(31):4571-4579.
- Wawra et al. Extended analyses of the Wnt/beta-catenin pathway: robustness and oscillatory behaviour.FEBS Letters. (2007), 581(21):4043-4048.
