Cookson et al., (2011). Queueing up for enzymatic processing: correlated signaling through coupled degradation.
April 2012, model of the month by Benedetta Frida Baldi
Original model: BIOMD0000000405
| In the present era of proteomic and gene expression analysis, we assume that there exist a direct coupling mechanism involved in a system where there is a correlated response of two entities that happen due to some kind of perturbation. In most cases this is true, but Cookson et al. (2011) [1, BIOMD0000000405] have pointed out that, a direct coupling could not always imply a strong signal correlation. For example, if two independent networks share a component in their pathways, then this simple and quite common fact lead to a significant cross-talk between the two networks. Cookson et al. (2011) used queuing theory, modelling and synthetic biology, to study this phenomenon quantitatively. Queuing theory is the study of waiting lines, positioning in the queue, and serving times in front of the queue. So far, this theory has been extensively used in telecommunications and computing, but not in the study of biological processes. | 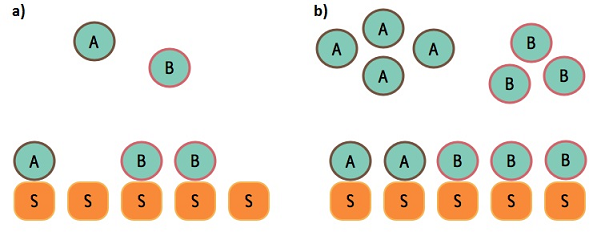 Figure 1: a) Underloaded system in queuing theory. The number of servers S is higher than the sum of the customers A and B. No customer is waiting to be served. b) Overloaded system in queuing theory. The number of customers is higher than the number of servers. Both A and B need to queue in order to be served by S. |
 Figure 2: a) Schematic representation of the computational model presented in the paper. Protein X1 and X2 are processed by the same enzyme E, forming the complex E1 and E2. X1 and X2 are produced and degraded irreversibly. b) Schematic representation of the stress response to nutrient starvation in E. coli. The sigma factor (σs) is produced at constant rate and degraded by ClpXP. During stress condition, like starvation, mistranslated proteins are produced and degraded by the same enzyme. The competition for ClpXP create an increase in σs concentration that provoke a stress response. Figure (b) is taken from [1]. | Considering two different type of customers A and B, that need to be served by server S, three scenarios are possible. (1) Underloaded system: In this case, the number of servers S is much higher than the customers A and B. So, neither of them need to wait or queue in order to be served(figure 1). (2) Overloaded system: In this case, the number of servers S is lower than the number of customers (A and B). As the consequence, the system become overloaded and A and/or B need to queue before being served (figure 2). (3) Finally, the third scenario take place when an equilibrium is reached. All the servers are occupied but no customers are waiting, since the serving time and new customer arrival are perfectly balanced. In order to prove that these dynamics can be observed in biological processes, Cookson et al. (2011) studied the stress response to nutrient starvation in Escherichia coli, characterised by a rapid and adaptive response to saturation of downstream enzymes. One of the most important enzyme in this network is ClpXP, an ATP-protease member of the AAA+ family (ATPases associated with various cellular activities). ClpXP consists of a hexameric ClpX ATPase and a tetradecameric ClpP serine peptidase and catalyse the degradation of mistranslated proteins and sigma factor (σs), the master stress regulator in E.coli (figure 3). In low stress conditions, the sigma factor is maintained at low level by ClpXP degradation, and consequently no stress response is triggered. However, during starvation, the amount of mistranslated proteins increases dramatically and they compete for ClpXP degradation. This competition increases the half-life of the sigma factor, which rises in concentration, and triggers the stress response. |
| The model deposited in Biomodel Database BIOMD0000000405] can accurately reproduce this behaviour. Two proteins X1 and X2 interact with the same enzyme E, to form E1 and E2 respectively (figure 4). X1 and X2 are produced at a constant rate, but also degraded to mimic the dilution effect due to cell division. In the stress response context, E represents ClpXP and X1 and X2 represent mistranslated protein and the sigma factor respectively. As shown in figure 5, the amount of mistranslated protein (derived from the starvation conditions) that is needed for triggering the accumulation of the sigma factor is highly correlated to its basal production rate (λσs). The higher the rate, lower the amount of mistranslated proteins that is required to cross the balance point (represented in Figure 5 as dotted lines). The application of queuing theory can exhaustively explain this sensitive response as an emerging property of the system, without the need of additional assumptions such as some type of strong cooperativity, a direct coupling (e.g. gene expression coordination), or protein-protein interactions. |  Figure 3: a) Result from the stochastic queuing model. Mean state-state level of the sigma factor (σs) related to the stress level represented as production rate of mistranslated proteins. The plot shows a sensitive response of the system, once the balance point (shown as a dotted line) has been crossed. Different sigma factor’s production rate λσs are indicated in the figure. b) Schematic representation of the synthetic circuit. YFP is produced by the PLtetO-1 promoter, which is repressed by TetR in the absence of doxycycline. CFP is produced by the Plac/ara-1 promoter, which is activated by AraC in the presence of arabinose. Both CFP and YFP molecules are tagged with identical LAA tags and are targeted for degradation by the ClpXP complexes. The figures (a) and (b) are taken from [1]. |
 Figure 4: a) Dynamic behaviour of the synthetic network on two-colour flow cytometry. A population of E.coli has been monitored under periodic series of doxycycline (in red) and the total fluorescence of YFP (green) and CFP (blue) integrated over the entire colony. b) Single cell trajectories two-colour flow cytometry. Single cells have been monitored under periodic series of doxycycline (in red) and the total fluorescence of YFP (green) and CFP (blue) are registered. The figures (a) and (b) are taken from [1]. | To further confirm the biological importance of the queuing analysis approach, the paper also presents a synthetic system to overexpress two different tagged proteins from promoters that are separate and uncorrelated, but both degraded by ClpXP (figure 6). Two-colour flow cytometry was used to monitor the dynamics of the signalling network. A series of doxycycline (Dox) pulses were applied to a large population of E.coli and the total YFP and CFP fluorescence were recorded and integrated over the entire colony. As shown in figure 7, the Dox pulses not only correlate well with YFP (which is expected, because Dox releases the inhibition on its promoter) but also with CFP. A similar trend was reported for single cell trajectories (figure 8). Based on these results, Cookson et al. (2011) conclude that the use of degradation tags can generate synchronisation among independent circuits in synthetic biology, and specifically enhance desired behaviours. |
Bibliographic References
- Cookson NA, Mather WH, Danino T, Mondragón-Palomino O, Williams RJ, Tsimring LS, Hasty J. Queueing up for enzymatic processing: correlated signaling through coupled degradation. Mol Syst Biol. Dec 20(7):561, 2011. [CiteXplore]
