Stefan et al. (2008), Allosteric Model of Calmodulin
March 2009, model of the month by Lu Li
Original model: BIOMD0000000183
| Long term potentiation (LTP) and long term depression (LTD) are the two most studied forms of synaptic plasticity, implicated in learning and memory [1]. They are both triggered by calcium, through the activation of calmodulin. This ubiquitous calcium buffer protein then activates calcineurin (PP2B) and calmodulin dependent protein kinase II (CaMKII), inducing LTD and LTP, respectively. Thus, the intriguing question is how calmodulin distinguishes between the two events. There are many computational models for calmodulin. However, none of these attempts to address this question in a mechanistic way. In the model presented here (BIOMD0000000183), Stefan et al., by constructing a detailed allosteric model of calmodulin, not only reconcile its different properties, but also demonstrate that it can activate PP2B and CaMKII in response to different calcium levels [2]. This model takes into account of two states of calmodulin, the open, active state (R) and the closed, inactive state (T). In either state, calmodulin can bind up to four calcium ions. The main idea is that calcium saturation is not necessary for the activation of calmodulin, but binding of calcium progressively lowers the free energy of the open state. As a consequence, calcium ions cooperatively bind to calmodulin, facilitating its transition from T to R. Once calmodulin becomes active, it binds to the targets proteins, PP2B and CaMKII. Figure 1 shows a fraction of this model: The illustrated reactions only concern calmodulin without (T0, R0) or with four calcium ions bound (TABCD, RABCD). There are several allosteric parameters that are important for this model: the allosteric isomerisation constant (L), the microscopic dissociation constants for R state (KiR) and the ratio of of R and T affinity (c). In order to obtain these parameters, the authors explored large parameter spaces using experimental data [3, 4]. The model is then validated by comparing simulation results with another set of experimental data reported by Crouch and Klee [5], as shown in Figure 2. |  Figure 1: Part of the reaction scheme used in this allosteric model. Taken from [2]. |
It has been observed that target binding increases the affinity between calmodulin and calcium ions. To demonstrate this, the authors set up four models, one of which contains only the R state of calmodulin; the second one only the T state; the third one both states without target, and the fourth one both states and the binding of CaMKII to the R state. As indicated in Figure 3, R state calmodulin has a sharper response to low concentrations of calcium because of higher affinity compared to the T state. Most importantly, upon binding CaMKII, the activation of calmodulin in response to different calcium concentrations, shifts towards the response obtained when only R state is considered. This means that target binding stabilises the open state of calmodulin.
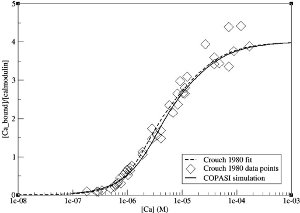 Figure 2: Comparison between simulation results (solid line) and experimental observations by Crouch and Klee [5] (diamonds). Figure taken from [2]. | 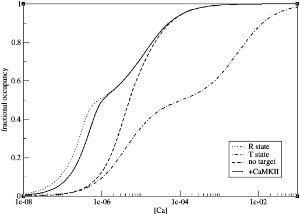 Figure 3: Increased affinity between calmodulin and calcium upon CaMKII binding. Figure taken from [2]. |  Figure 4: Differential activation of PP2B and CaMKII at different calcium concentrations. Figure taken from [2]. |
When there is both PP2B and CaMKII in the system, authors reveal that the activation of the two targets by calmodulin is differential, dependent on calcium concentrations. As illustrated in Figure 4, low calcium concentration results in a higher percentage of PP2B than CaMKII being activated. Even when calcium concentration reaches the level that fully activates PP2B, less than ten percent of CaMKII is activated. This is because low calcium concentration results in a limited number of R state calmodulin, which preferentially binds to the higher affinity target, PP2B [6,7]. But this binding also stabilises the R state, encouraging more calmodulin to become open once larger amount of calcium ions are available. This might be the reason that the response of CaMKII to calcium concentration change is steeper than PP2B. To summarise, a moderate elevation of calcium activates PP2B, while a higher increase activates CaMKII, a phenomenon which has already been suggested by Lisman [8].
This model gives accurate explanations of calmodulin activation and functions, which cannot be satisfactorily depicted by sequential models or models using the Hill equation or Adair function. Therefore, this model provides a base for building more complicated models to explore the molecular mechanisms of synaptic plasticity.
Bibliographic References
- S.J. Martin, P.D. Grimwood, and R.G. Morris. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649-711, 2000. [SRS@EBI]
- M.I. Stefan, S.J. Edelstein, and N. Le Novère. An allosteric model of calmodulin explains differential activation of PP2B and CaMKII. Proc Natl Acad Sci U S A 105(31):10768-10773, 2008. [SRS@EBI] [Erratum]
- J.M. Shifman, M.H. Choi, S. Mihalas, S.L. Mayo, and M.B. Kennedy. Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by calmodulin with two bound calciums. Proc Natl Acad Sci U S A 103(38):13968-13973, 2006. [SRS@EBI]
- O.B. Peersen, T.S. Madsen, and J.J. Falke. Intermolecular tuning of calmodulin by target peptides and proteins: differential effects on Ca2+ binding and implications for kinase activation. Protein Sci. 6(4):794-807, 1997. [SRS@EBI]
- T.H. Crouch, C.B. Klee. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry 19(16):3692-3698, 1980. [SRS@EBI]
- A.R. Quintana, D. Wang, J.E. Forbes, and M.N. Waxham. Kinetics of calmodulin binding to calcineurin. Biochem Biophys Res Commun 334(2):674-680, 2005. [SRS@EBI]
- A. Tzortzopoulos, K. Török. Mechanism of the T286A-mutant alphaCaMKII interactions with Ca2+/calmodulin and ATP. Biochemistry 43(21):6404-6414, 2004. [SRS@EBI]
- J. Lisman. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A 86(23):9574-9578, 1989. [SRS@EBI]
